Building future
worth through data
Creating future worth through data.
worth through data
Total 137 Posts
-
 Launch of functional health supplement brand ‘DiNU’The company launched its health supplement brand DiNU (Define Nutrition) on August 17. Under the slogan “The most ideal health supplement focused on functionality,” the DiNU product line is now available for purchase on Naver Smart Store. Based on the company’s pharmaceutical identity, the DiNU brand emphasizes product functionality. It began with the release of DiNU Propolis, a chewable tablet focused on oral antibacterial benefits, and plans to sequentially launch additional products this year—including probiotics that survive through the intestines and high-absorption collagen products. In addition, the company will directly supply 100% ascorbic acid and 100% MSM products, which are currently sold in hospitals, through its online store. In line with its commitment to sustainability, DiNU has adopted Earth Pack, a 100% biodegradable packaging material, and uses eco-friendly materials for packaging and shipping. The company also minimizes printed materials such as manuals and brochures, opting instead for digital content to further expand its environmental consideration. Going forward, DiNU aims to redefine the concept of nutrition through formulation and ingredient development focused on functionality. The brand plans to deliver various functional health supplements directly to consumers and engage them through diverse promotional events that make the brand approachable and familiar.2021.08.24
Launch of functional health supplement brand ‘DiNU’The company launched its health supplement brand DiNU (Define Nutrition) on August 17. Under the slogan “The most ideal health supplement focused on functionality,” the DiNU product line is now available for purchase on Naver Smart Store. Based on the company’s pharmaceutical identity, the DiNU brand emphasizes product functionality. It began with the release of DiNU Propolis, a chewable tablet focused on oral antibacterial benefits, and plans to sequentially launch additional products this year—including probiotics that survive through the intestines and high-absorption collagen products. In addition, the company will directly supply 100% ascorbic acid and 100% MSM products, which are currently sold in hospitals, through its online store. In line with its commitment to sustainability, DiNU has adopted Earth Pack, a 100% biodegradable packaging material, and uses eco-friendly materials for packaging and shipping. The company also minimizes printed materials such as manuals and brochures, opting instead for digital content to further expand its environmental consideration. Going forward, DiNU aims to redefine the concept of nutrition through formulation and ingredient development focused on functionality. The brand plans to deliver various functional health supplements directly to consumers and engage them through diverse promotional events that make the brand approachable and familiar.2021.08.24 -
 Achieved 13.9 billion won in operating profit for the first half, up 13% year-on-yearThe company announced that its accumulated operating profit for the first half of 2021 reached 13.9 billion won, marking a 13% increase year-on-year. Revenue rose 13% to 83.8 billion won, while net profit grew 19% to 8.9 billion won. Strong performance continued in the second quarter, with revenue of 44.2 billion won, up 14% from the same period last year. Operating profit increased 8% to 7.2 billion won, and quarterly net profit climbed 22% to 4.7 billion won. The company attributed its strong results to a focused sales strategy centered on key product lines. Robust sales were reported for Liporase Injection (a wellness injection), Thymosin Injection and Vita d Injection (immune boosters), as well as new and exclusive products including the dyslipidemia combination drug “Newtozet,” the fat absorption inhibitor “Zero be,” and the carbohydrate absorption inhibitor “Migbos Film-Coated Tablet,” which is exclusively marketed in Korea. In the second half of the year, the company plans to launch its new health supplement brand “DiNU (Define Nutrition)”, built on its pharmaceutical expertise and carrying the slogan “The most ideal health supplement focused on functionality.” New products will roll out sequentially from late August. Sales of injection products and newly launched items continue to grow steadily, and the company plans to promote “DiNU” aggressively to ensure a successful market entry and further drive revenue growth. Additionally, the relocation of the Central Research Center and Bio Research Center to the Pangyo Industry-Academia Cooperation Center has strengthened the company’s R&D pipeline, underscoring its evolution into a research-driven pharmaceutical enterprise.2021.08.13
Achieved 13.9 billion won in operating profit for the first half, up 13% year-on-yearThe company announced that its accumulated operating profit for the first half of 2021 reached 13.9 billion won, marking a 13% increase year-on-year. Revenue rose 13% to 83.8 billion won, while net profit grew 19% to 8.9 billion won. Strong performance continued in the second quarter, with revenue of 44.2 billion won, up 14% from the same period last year. Operating profit increased 8% to 7.2 billion won, and quarterly net profit climbed 22% to 4.7 billion won. The company attributed its strong results to a focused sales strategy centered on key product lines. Robust sales were reported for Liporase Injection (a wellness injection), Thymosin Injection and Vita d Injection (immune boosters), as well as new and exclusive products including the dyslipidemia combination drug “Newtozet,” the fat absorption inhibitor “Zero be,” and the carbohydrate absorption inhibitor “Migbos Film-Coated Tablet,” which is exclusively marketed in Korea. In the second half of the year, the company plans to launch its new health supplement brand “DiNU (Define Nutrition)”, built on its pharmaceutical expertise and carrying the slogan “The most ideal health supplement focused on functionality.” New products will roll out sequentially from late August. Sales of injection products and newly launched items continue to grow steadily, and the company plans to promote “DiNU” aggressively to ensure a successful market entry and further drive revenue growth. Additionally, the relocation of the Central Research Center and Bio Research Center to the Pangyo Industry-Academia Cooperation Center has strengthened the company’s R&D pipeline, underscoring its evolution into a research-driven pharmaceutical enterprise.2021.08.13 -
 Achieved 6.6 billion won in operating profit for Q1, up 17% year-on-yearThe company continued its growth momentum in 2021. In the first quarter of 2021, it achieved an operating profit of 6.6 billion won, representing a 17% increase year-on-year. Revenue rose 11% to 39.4 billion won, while net profit also increased 10% to 4 billion won. The company attributed its overall performance improvement to the success of its strategic focus on core product lines and systematic distribution network management, both of which have been in place since the previous year. Steady results continue through the company’s management efficiency strategy, which includes proactive risk management measures. Sales of Zero be Capsule, a fat absorption inhibitor launched in 2020, and Avatri Tablet, a triple combination drug for hypertension and hyperlipidemia, have been steadily growing. In 2021, the company further accelerated growth with the domestically exclusive launch of “Migbos Film-Coated Tablet.” Increased sales of core products and improvements in the distribution structure continue to drive sustainable performance growth. With the completion of the company’s Industry-Academia Research Center in Pangyo Second Techno Valley, its R&D capabilities have been further strengthened, enabling the development of a broader range of products to sustain long-term growth.2021.05.12
Achieved 6.6 billion won in operating profit for Q1, up 17% year-on-yearThe company continued its growth momentum in 2021. In the first quarter of 2021, it achieved an operating profit of 6.6 billion won, representing a 17% increase year-on-year. Revenue rose 11% to 39.4 billion won, while net profit also increased 10% to 4 billion won. The company attributed its overall performance improvement to the success of its strategic focus on core product lines and systematic distribution network management, both of which have been in place since the previous year. Steady results continue through the company’s management efficiency strategy, which includes proactive risk management measures. Sales of Zero be Capsule, a fat absorption inhibitor launched in 2020, and Avatri Tablet, a triple combination drug for hypertension and hyperlipidemia, have been steadily growing. In 2021, the company further accelerated growth with the domestically exclusive launch of “Migbos Film-Coated Tablet.” Increased sales of core products and improvements in the distribution structure continue to drive sustainable performance growth. With the completion of the company’s Industry-Academia Research Center in Pangyo Second Techno Valley, its R&D capabilities have been further strengthened, enabling the development of a broader range of products to sustain long-term growth.2021.05.12 -
 Completion of Industry-Academia Research Center in Pangyo Second Techno ValleyDaehan New Pharm has completed the construction of its Industry-Academia Research Center in Pangyo Second Techno Valley. The newly built Daehan New Pharm Industry-Academia Research Center, located near the Daewangpangyo Interchange, spans from five basement levels to nine floors above ground and has been designed to become a new landmark in the area. The center was established with the themes of “research and development” and “communication” to enhance work efficiency and employee welfare. The basement and the first and second floors house lobbies, a main auditorium, and seminar rooms, providing spaces for collaboration. To maximize synergy among its pharmaceutical, veterinary, and bio divisions, the company relocated its Central Research Center from Hyangnam and its Bio Research Center from Osong to Pangyo. The new facility also lays the groundwork for open innovation. Daehan New Pharm will form a consortium with Semyung University’s and University of Suwon’s affiliated research institutes, which will jointly occupy the center. The company plans to continuously promote industry-academia collaboration through these partnerships. The establishment of the Daehan New Pharm Industry-Academia Research Center marks a significant milestone in the company’s growth as a pharmaceutical and biotechnology enterprise. Internally, it will serve as a workplace that fosters employee pride and engagement.2021.04.20
Completion of Industry-Academia Research Center in Pangyo Second Techno ValleyDaehan New Pharm has completed the construction of its Industry-Academia Research Center in Pangyo Second Techno Valley. The newly built Daehan New Pharm Industry-Academia Research Center, located near the Daewangpangyo Interchange, spans from five basement levels to nine floors above ground and has been designed to become a new landmark in the area. The center was established with the themes of “research and development” and “communication” to enhance work efficiency and employee welfare. The basement and the first and second floors house lobbies, a main auditorium, and seminar rooms, providing spaces for collaboration. To maximize synergy among its pharmaceutical, veterinary, and bio divisions, the company relocated its Central Research Center from Hyangnam and its Bio Research Center from Osong to Pangyo. The new facility also lays the groundwork for open innovation. Daehan New Pharm will form a consortium with Semyung University’s and University of Suwon’s affiliated research institutes, which will jointly occupy the center. The company plans to continuously promote industry-academia collaboration through these partnerships. The establishment of the Daehan New Pharm Industry-Academia Research Center marks a significant milestone in the company’s growth as a pharmaceutical and biotechnology enterprise. Internally, it will serve as a workplace that fosters employee pride and engagement.2021.04.20 -
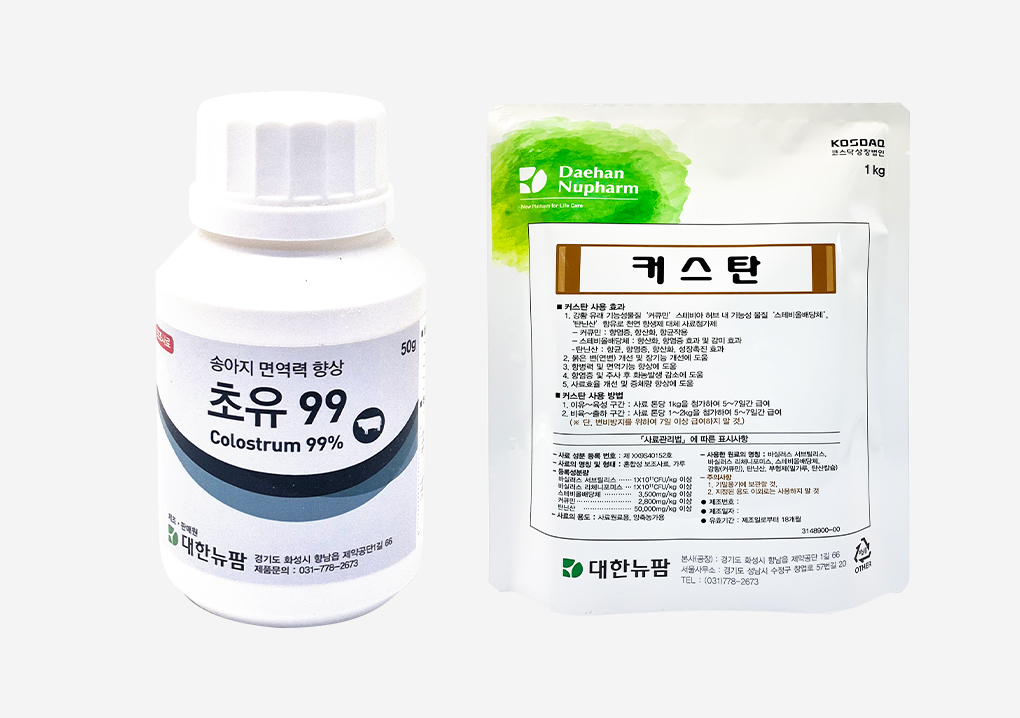 Launch of livestock disease-prevention feed supplements ‘Choyu 99’ and ‘Custan’Daehan New Pharm Co., Ltd. has launched two new livestock feed supplements, “Choyu 99” and “Custan,” designed to help prevent livestock diseases. Choyu 99 is a high-purity colostrum-based probiotic complex that promotes intestinal health in calves. The company signed a supply agreement with a leading U.S. colostrum distributor known for its premium-grade colostrum, which contains high levels of IgA (Immunoglobulin A) and IgG (Immunoglobulin G). With a purity of 99.8%, Choyu 99 provides the immune benefits of colostrum in a fast and convenient way. Colostrum feeding is recognized as the most essential factor in preventing diseases in newborn calves. Immune substances that prevent diarrhea can only be delivered through colostrum, which must be administered within 24 hours after birth. Although calves need colostrum equivalent to more than 6% of their body weight, most mother cows do not produce sufficient quantities. Therefore, feeding frozen colostrum or colostrum-based supplements has become indispensable. Custan is a natural complex formulation combining highly bioavailable curcumin with special-coated tannic acid, designed to enhance immunity and support antiviral, antibacterial, anti-inflammatory, and productivity-improving effects. Trials in growing and finishing pigs—particularly those difficult to treat during antibiotic withdrawal periods—showed that Custan significantly improved diarrhea symptoms. Moreover, when administered before and after vaccination, including foot-and-mouth disease shots, it significantly reduced abscess formation. Numerous studies and case reports have confirmed that enhanced immunity and disease resistance contribute greatly to improving productivity across various swine production stages. As a combination of these two active ingredients, Custan can deliver maximum effects even with short-term administration (5–7 days), depending on the farm’s conditions. The company stated its commitment to continued collaboration and joint development with technological partners, aiming to contribute to the livestock and related industries as a sustainable and socially responsible enterprise that pursues shared growth.2021.02.23
Launch of livestock disease-prevention feed supplements ‘Choyu 99’ and ‘Custan’Daehan New Pharm Co., Ltd. has launched two new livestock feed supplements, “Choyu 99” and “Custan,” designed to help prevent livestock diseases. Choyu 99 is a high-purity colostrum-based probiotic complex that promotes intestinal health in calves. The company signed a supply agreement with a leading U.S. colostrum distributor known for its premium-grade colostrum, which contains high levels of IgA (Immunoglobulin A) and IgG (Immunoglobulin G). With a purity of 99.8%, Choyu 99 provides the immune benefits of colostrum in a fast and convenient way. Colostrum feeding is recognized as the most essential factor in preventing diseases in newborn calves. Immune substances that prevent diarrhea can only be delivered through colostrum, which must be administered within 24 hours after birth. Although calves need colostrum equivalent to more than 6% of their body weight, most mother cows do not produce sufficient quantities. Therefore, feeding frozen colostrum or colostrum-based supplements has become indispensable. Custan is a natural complex formulation combining highly bioavailable curcumin with special-coated tannic acid, designed to enhance immunity and support antiviral, antibacterial, anti-inflammatory, and productivity-improving effects. Trials in growing and finishing pigs—particularly those difficult to treat during antibiotic withdrawal periods—showed that Custan significantly improved diarrhea symptoms. Moreover, when administered before and after vaccination, including foot-and-mouth disease shots, it significantly reduced abscess formation. Numerous studies and case reports have confirmed that enhanced immunity and disease resistance contribute greatly to improving productivity across various swine production stages. As a combination of these two active ingredients, Custan can deliver maximum effects even with short-term administration (5–7 days), depending on the farm’s conditions. The company stated its commitment to continued collaboration and joint development with technological partners, aiming to contribute to the livestock and related industries as a sustainable and socially responsible enterprise that pursues shared growth.2021.02.23 -
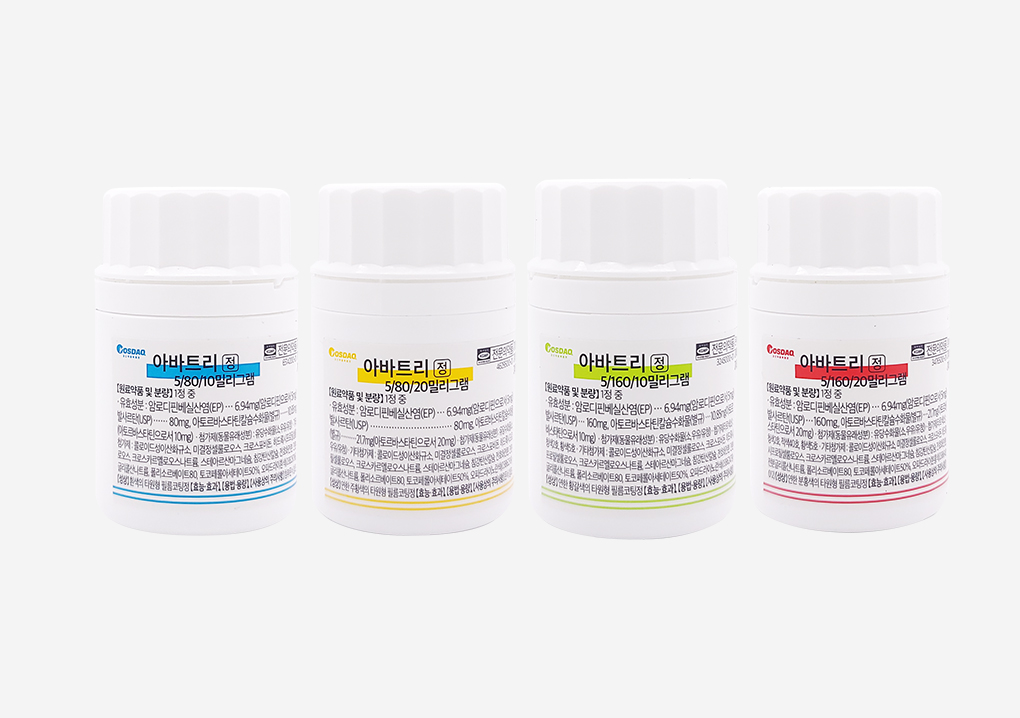 Launch of triple combination drug for hypertension and hyperlipidemia ‘Avatri Tablet’Daehan New Pharm has launched “Avatri Tablet,” the first triple combination drug in Korea containing atorvastatin, amlodipine, and valsartan. Avatri Tablet is designed to offer enhanced medication convenience for patients with hypertension and dyslipidemia. According to data from the Health Insurance Review and Assessment Service (HIRA), about 31.9% of hypertensive patients receive concurrent treatment for dyslipidemia, and the number of such comorbid cases is rapidly increasing. Combination therapies such as Avatri are known to improve long-term treatment adherence in hypertension and dyslipidemia management. Compared with patients taking separate medications, those on fixed-dose combination therapies had a 117% lower risk of discontinuation, with an average treatment duration of 35 months versus 7 months for separate dosing. Additionally, the UMPIRE study confirmed that fixed-dose combination therapy improves medication adherence by 33%. The efficacy of Avatri Tablet in controlling seated systolic blood pressure (siSBP) and low-density lipoprotein cholesterol (LDL-C) was verified through clinical trials. After eight weeks of administration, the Avatri group showed a 22.75 mmHg reduction in siSBP, compared to a 5.4 mmHg reduction in the valsartan/atorvastatin comparator group. In lipid control, Avatri also demonstrated remarkable results. After eight weeks, the Avatri -treated group showed a 47.15% reduction in LDL-C, whereas the control group receiving amlodipine/valsartan showed an increase in LDL-C levels. By combining three active ingredients to treat two diseases, Avatri provides effective regulation of lipid and blood pressure levels, making it highly beneficial for preventing cardiovascular diseases in hypertensive patients with dyslipidemia who require intensive treatment and management. The company stated that Avatri has strong potential for development in various forms, including those aimed at improving medication adherence, and reaffirmed its commitment to developing improved new drugs with superior therapeutic efficacy.2021.02.05
Launch of triple combination drug for hypertension and hyperlipidemia ‘Avatri Tablet’Daehan New Pharm has launched “Avatri Tablet,” the first triple combination drug in Korea containing atorvastatin, amlodipine, and valsartan. Avatri Tablet is designed to offer enhanced medication convenience for patients with hypertension and dyslipidemia. According to data from the Health Insurance Review and Assessment Service (HIRA), about 31.9% of hypertensive patients receive concurrent treatment for dyslipidemia, and the number of such comorbid cases is rapidly increasing. Combination therapies such as Avatri are known to improve long-term treatment adherence in hypertension and dyslipidemia management. Compared with patients taking separate medications, those on fixed-dose combination therapies had a 117% lower risk of discontinuation, with an average treatment duration of 35 months versus 7 months for separate dosing. Additionally, the UMPIRE study confirmed that fixed-dose combination therapy improves medication adherence by 33%. The efficacy of Avatri Tablet in controlling seated systolic blood pressure (siSBP) and low-density lipoprotein cholesterol (LDL-C) was verified through clinical trials. After eight weeks of administration, the Avatri group showed a 22.75 mmHg reduction in siSBP, compared to a 5.4 mmHg reduction in the valsartan/atorvastatin comparator group. In lipid control, Avatri also demonstrated remarkable results. After eight weeks, the Avatri -treated group showed a 47.15% reduction in LDL-C, whereas the control group receiving amlodipine/valsartan showed an increase in LDL-C levels. By combining three active ingredients to treat two diseases, Avatri provides effective regulation of lipid and blood pressure levels, making it highly beneficial for preventing cardiovascular diseases in hypertensive patients with dyslipidemia who require intensive treatment and management. The company stated that Avatri has strong potential for development in various forms, including those aimed at improving medication adherence, and reaffirmed its commitment to developing improved new drugs with superior therapeutic efficacy.2021.02.05 -
 Simplification of investment structureThe company had indirectly held shares of Caspian Sunrise Plc, a Kazakh oil field operator based in the United Kingdom, through Baverstock GmbH. However, as part of efforts to simplify its investment structure, the company is currently in the process of liquidating Baverstock GmbH, through which it has now come to directly hold 224,830,964 shares of Caspian Sunrise Plc.2021.01.05
Simplification of investment structureThe company had indirectly held shares of Caspian Sunrise Plc, a Kazakh oil field operator based in the United Kingdom, through Baverstock GmbH. However, as part of efforts to simplify its investment structure, the company is currently in the process of liquidating Baverstock GmbH, through which it has now come to directly hold 224,830,964 shares of Caspian Sunrise Plc.2021.01.05 -
 Patent registration for a stem cell storage excipient compositionDaehan New Pharm's Bio Research Center registered a patent on December 11 for a composition method of excipients that increase the storage period and survival rate of stem cells. Cell therapies achieve therapeutic effects by replacing damaged tissues/organs directly or through indirect actions via paracrine and endocrine factors, with ongoing research targeting various intractable diseases. Adult stem cells, especially adipose-derived mesenchymal stem cells, are advantageous due to ethical and safety benefits and ease of isolation with high cell yields. Unlike conventional drugs, cell therapies consist of living cells, resulting in a very short finished product shelf life of 1–2 days. Cell survival rates during transportation critically affect drug quality, directly limiting market competitiveness. To address these challenges, buffers to increase cell viability and rapid thawing methods post-freezing have been developed. However, toxicity and sterility issues related to commonly used DMSO freezing agents remain problematic. Daehan New Pharm developed a composition based on approved safe substances to extend storage duration and enhance cell survival rates for finished cell therapy products. Candidate substances including ascorbic acid, PDRN, pyridoxine, and DMEA wereed, and stem cell survival was evaluated over storage periods alongside analysis of post-storage stem cell characteristics. While single treatments of candidates had limited impact, a mixed composition containing ascorbic acid as the base with PDRN, pyridoxine, and DMEA significantly increased cell survival. Cell function was preserved even after more than three days of storage, as confirmed by flow cytometry and differentiation ability tests. This technology is expected to greatly extend the conventional 24-hour storage period during distribution in commercial stem cell therapies, enhancing product value and competitiveness. It is applicable not only to animal stem cells but also to human stem cells, with potential integration into various products. The Bio Research Center is dedicated to the efficient culturing, process development, transportation, and storage of stem cells, supporting the commercialization of stem cell therapies.2020.12.23
Patent registration for a stem cell storage excipient compositionDaehan New Pharm's Bio Research Center registered a patent on December 11 for a composition method of excipients that increase the storage period and survival rate of stem cells. Cell therapies achieve therapeutic effects by replacing damaged tissues/organs directly or through indirect actions via paracrine and endocrine factors, with ongoing research targeting various intractable diseases. Adult stem cells, especially adipose-derived mesenchymal stem cells, are advantageous due to ethical and safety benefits and ease of isolation with high cell yields. Unlike conventional drugs, cell therapies consist of living cells, resulting in a very short finished product shelf life of 1–2 days. Cell survival rates during transportation critically affect drug quality, directly limiting market competitiveness. To address these challenges, buffers to increase cell viability and rapid thawing methods post-freezing have been developed. However, toxicity and sterility issues related to commonly used DMSO freezing agents remain problematic. Daehan New Pharm developed a composition based on approved safe substances to extend storage duration and enhance cell survival rates for finished cell therapy products. Candidate substances including ascorbic acid, PDRN, pyridoxine, and DMEA wereed, and stem cell survival was evaluated over storage periods alongside analysis of post-storage stem cell characteristics. While single treatments of candidates had limited impact, a mixed composition containing ascorbic acid as the base with PDRN, pyridoxine, and DMEA significantly increased cell survival. Cell function was preserved even after more than three days of storage, as confirmed by flow cytometry and differentiation ability tests. This technology is expected to greatly extend the conventional 24-hour storage period during distribution in commercial stem cell therapies, enhancing product value and competitiveness. It is applicable not only to animal stem cells but also to human stem cells, with potential integration into various products. The Bio Research Center is dedicated to the efficient culturing, process development, transportation, and storage of stem cells, supporting the commercialization of stem cell therapies.2020.12.23 -
 The 3rd quarter revenue reached 113.7 billion won, marking a 15% increase year-on-yearIn 3Q 2020, Daehan New Pharm achieved a cumulative operating profit of 19.6 billion won with an operating margin of 17%, representing a 21% increase compared to the same period last year. Revenue grew 15% to 113.7 billion won, and net profit reached 6.3 billion won. The quarterly performance also showed growth momentum. Operating profit for 3Q rose 40% year-on-year to 7.4 billion won, while revenue increased 23% to 39.5 billion won. The growth was driven by a broad increase in product sales. The company launched "Fine S Tablet," a cold and allergic rhinitis medicine with bromelain as the main ingredient, and expanded sales of "Luthione Tablet," containing glutathione, well known for its use in whitening injections. Moreover, despite the challenges posed by the COVID-19 situation, the company has actively pursued research and development efforts. This overview reflects Daehan New Pharm’s strong financial performance and commitment to innovation during this period.2020.11.11
The 3rd quarter revenue reached 113.7 billion won, marking a 15% increase year-on-yearIn 3Q 2020, Daehan New Pharm achieved a cumulative operating profit of 19.6 billion won with an operating margin of 17%, representing a 21% increase compared to the same period last year. Revenue grew 15% to 113.7 billion won, and net profit reached 6.3 billion won. The quarterly performance also showed growth momentum. Operating profit for 3Q rose 40% year-on-year to 7.4 billion won, while revenue increased 23% to 39.5 billion won. The growth was driven by a broad increase in product sales. The company launched "Fine S Tablet," a cold and allergic rhinitis medicine with bromelain as the main ingredient, and expanded sales of "Luthione Tablet," containing glutathione, well known for its use in whitening injections. Moreover, despite the challenges posed by the COVID-19 situation, the company has actively pursued research and development efforts. This overview reflects Daehan New Pharm’s strong financial performance and commitment to innovation during this period.2020.11.11


 Home
Home